This new multidisciplinary guideline is proposed to address biopharmaceutics classification system (BCS)-based biowaivers. BCS-based biowaivers may be applicable to BCS Class I and III drugs, however BCS-based biowaivers for these two classes are not recognized worldwide. This means that pharmaceutical companies have to follow different approaches in the different regions. This guideline will provide recommendations to support the biopharmaceutics classification of medicinal products and will provide recommendations to support the waiver of bioequivalence studies. This will result in the harmonisation of current regional guidelines/guidance and support streamlined global drug development.
Keywords: Bioequivalence study exemptions, solubility, permeability, in vitro dissolution
BCS Class II drugs are discusse d in this article. With emphasis on the solid dispersion. Technique and i ts app lication. Formu lation of. Solid disp ersion in water-soluble carriers has. A drug product is eligible for a BCS-based biowaiver provid that the drug substance(s) satisfy the ed criteria regarding solubility and permeability (BCS Class I and III), the drug product is an immediate-release oral dosage form with systemic action, and the drug product is dosage the same form and strength as the reference product.

The BCS classification system is used to categorize drugs and serves to help anticipate whether drugs will have bioavailability/ bioequivalence problems. BCS classifies drugs according to their solubility and permeability. A drug is considered to have high solubility if drug substance at the highest dose strength for an immediate release formulation can be dissolved in < 250 mL of water over a pH range of 1-7.5. A high permeability drug is one that has either complete intestinal absorption (fa > 90%) or exhibits rapid movement through intestinal epithelia cells in vitro. BCS classifies all drugs into four categories as shown in Table 3.3.
Table 3.3. Biopharmaceutics classification system
BCS CLASS I High solubility High permeability
BCS CLASS III High solubility Low permeability
BCS CLASS II Low solubility High permeability
BCS CLASS IV Low solubility Low permeability
BCS class I compounds (high solubility and permeability) are unlikely to show bioavailability/bioequivalence issues. Therefore, for BCS class I drugs, in vitro dissolution studies are thought to provide sufficient information to assure in vivo product performance making full in vivo bioavailability/bioequivalence studies unnecessary. BCS class II and III drugs are not eligible for biowavers due to anticipated formulation differences in oral exposure. BCS class IV compounds, in general, are problematic with both poor solubility and permeability. The following tables (see Tables 3.4-3.7) contain lists of drugs that are categorized as BCS classes I to IV.
Abacavir | Diazepam | Ketorolac | Phenobarbital |
Acetaminophen | Diltiazem | Ketoprofen | Phenylalanine |
Diphenhydramine | Labetolol | Prednisolone | |
Amiloride | Disopyramide | Primaquine | |
Amitryptyline | Doxepin | Levofloxacin | Promazine |
Antipyrine | Doxycycline | Lidocaine | Propranolol |
Atropine | Enalapril | Lomefloxacin | Quinidine |
Ephedrine | Meperidine | Rosiglitazone | |
Caffeine | Ergonovine | Metoprolol | Salicylic acid |
Captopril | Ethambutol | Metronidazole | |
Chloroquine | Ethinyl estradiol Kicad library location macon. | Midazolam | Valproic acid |
Chlorpheniramine | Fluoxetine | Minocycline | Verapamil |
Cyclophosphamide | Glucose | Misoprostol | Zidovudine |
Desipramine | Imipramine | Nifedipine |

Adapted from Wu and Benet (2005)
Adapted from Wu and Benet (2005)
Amiodarone | Diclofenac | Itraconazole | Piroxicam |
Atorvastatin | Diflunisal | Ketoconazole | Raloxifene |
Azithromycin | Digoxin | Lansoprazole | Ritonavir |
Carbamazepine | Erythromycin | Lovastatin | Saquinavir |
Carvedilol | Flurbiprofen | Mebendazole | Sirolimus |
Chlorpromazine | Glipizide | Naproxen | Spironolactone |
Cisapride | Glyburide | Nelfinavir | Tacrolimus |
Ciprofloxacin | Griseofulvin | Ofloxacin | Talinolol |
Cyclosporine | Ibuprofen | Oxaprozin | Tamoxifen |
Danazol | Indinavir | Phenazopyridine | Terfenadine |
Dapsone | Indomethacin | Phenytoin | Warfarin |
Adapted from Wu and Benet (2005)
Adapted from Wu and Benet (2005)
Table 3.6. BCS class III compounds (high solubility, low permeability)
Acyclovir
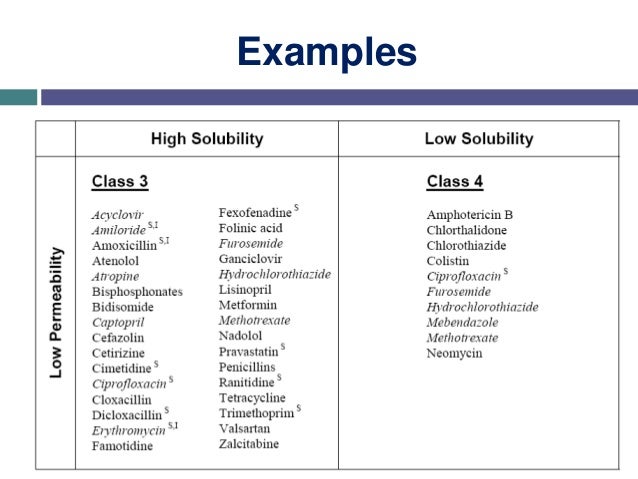
Amiloride
Amoxicillin
Atenolol
Atropine
Bisphosphonates
Bidisomide
Bsc Classification
Captopril
Cefazolin
Cetirizine
Cimetidine
Ciprofloxacin
Cloxacillin
Dicloxacillin
Erythromycin
Famotidine
Fexofenadine
Folinic acid
Furosemide
Ganciclovir
Hydrochlorothiazide
Lisinopril
Methotrexate
Nadolol
Pravastatin
Penicillins
Ranitidine
Tetracycline
Trimethoprim
Valsartan
Zalcitabine
Adapted from Wu and Benet (2005)

The BCS classification system is used to categorize drugs and serves to help anticipate whether drugs will have bioavailability/ bioequivalence problems. BCS classifies drugs according to their solubility and permeability. A drug is considered to have high solubility if drug substance at the highest dose strength for an immediate release formulation can be dissolved in < 250 mL of water over a pH range of 1-7.5. A high permeability drug is one that has either complete intestinal absorption (fa > 90%) or exhibits rapid movement through intestinal epithelia cells in vitro. BCS classifies all drugs into four categories as shown in Table 3.3.
Table 3.3. Biopharmaceutics classification system
BCS CLASS I High solubility High permeability
BCS CLASS III High solubility Low permeability
BCS CLASS II Low solubility High permeability
BCS CLASS IV Low solubility Low permeability
BCS class I compounds (high solubility and permeability) are unlikely to show bioavailability/bioequivalence issues. Therefore, for BCS class I drugs, in vitro dissolution studies are thought to provide sufficient information to assure in vivo product performance making full in vivo bioavailability/bioequivalence studies unnecessary. BCS class II and III drugs are not eligible for biowavers due to anticipated formulation differences in oral exposure. BCS class IV compounds, in general, are problematic with both poor solubility and permeability. The following tables (see Tables 3.4-3.7) contain lists of drugs that are categorized as BCS classes I to IV.
Abacavir | Diazepam | Ketorolac | Phenobarbital |
Acetaminophen | Diltiazem | Ketoprofen | Phenylalanine |
Diphenhydramine | Labetolol | Prednisolone | |
Amiloride | Disopyramide | Primaquine | |
Amitryptyline | Doxepin | Levofloxacin | Promazine |
Antipyrine | Doxycycline | Lidocaine | Propranolol |
Atropine | Enalapril | Lomefloxacin | Quinidine |
Ephedrine | Meperidine | Rosiglitazone | |
Caffeine | Ergonovine | Metoprolol | Salicylic acid |
Captopril | Ethambutol | Metronidazole | |
Chloroquine | Ethinyl estradiol Kicad library location macon. | Midazolam | Valproic acid |
Chlorpheniramine | Fluoxetine | Minocycline | Verapamil |
Cyclophosphamide | Glucose | Misoprostol | Zidovudine |
Desipramine | Imipramine | Nifedipine |
Adapted from Wu and Benet (2005)
Adapted from Wu and Benet (2005)
Amiodarone | Diclofenac | Itraconazole | Piroxicam |
Atorvastatin | Diflunisal | Ketoconazole | Raloxifene |
Azithromycin | Digoxin | Lansoprazole | Ritonavir |
Carbamazepine | Erythromycin | Lovastatin | Saquinavir |
Carvedilol | Flurbiprofen | Mebendazole | Sirolimus |
Chlorpromazine | Glipizide | Naproxen | Spironolactone |
Cisapride | Glyburide | Nelfinavir | Tacrolimus |
Ciprofloxacin | Griseofulvin | Ofloxacin | Talinolol |
Cyclosporine | Ibuprofen | Oxaprozin | Tamoxifen |
Danazol | Indinavir | Phenazopyridine | Terfenadine |
Dapsone | Indomethacin | Phenytoin | Warfarin |
Adapted from Wu and Benet (2005)
Adapted from Wu and Benet (2005)
Table 3.6. BCS class III compounds (high solubility, low permeability)
Acyclovir
Amiloride
Amoxicillin
Atenolol
Atropine
Bisphosphonates
Bidisomide
Bsc Classification
Captopril
Cefazolin
Cetirizine
Cimetidine
Ciprofloxacin
Cloxacillin
Dicloxacillin
Erythromycin
Famotidine
Fexofenadine
Folinic acid
Furosemide
Ganciclovir
Hydrochlorothiazide
Lisinopril
Methotrexate
Nadolol
Pravastatin
Penicillins
Ranitidine
Tetracycline
Trimethoprim
Valsartan
Zalcitabine
Adapted from Wu and Benet (2005)
Amphotericin B | Furosemide |
Chlorthalidone | Hydrochlorothiazide |
Chlorothiazide | Mebendazole |
Colistin | Methotrexate |
Ciprofloxacin | Neomycin |
Adapted from Wu and Benet (2005)
Adapted from Wu and Benet (2005)
Bcs Class 2 Drugs List
Continue reading here: Biopharmaceutics Drug Disposition Classification System BDDCS
Bcs Class 2 Anticancer Drug List
Was this article helpful?

